HRT and SERMs: New Guidelines for Patient Management - Part 1
Chairperson: Thomas E. Nolan, MD, MBA; Faculty: Robin K. Dore, MD; Lori Mosca,
MD, MPH, PhD; Jane Cauley, DrPH; Susan Johnson, MD
Editorial Content produced by the Annenberg Center for Health Sciences.
Copyright © 2003 Quadrant HealthCom Inc.
This CME activity, "HRT and SERMs: New Guidelines for
Patient Management," was originally offered as a supplement to the March
2002 Special Edition to The Female Patient, certified for CME. The original
supplement was published prior to the early termination of the estrogen/progestin
arm of the Women's Health Initiative (WHI) trial in July 2002 due to adverse
outcomes. The companion estrogen-alone trial continues, with close monitoring
by the data and safety monitoring board. The Women's International Study on
long Duration Oestrogen after Menopause (WISDOM) trial was halted in October
2002 because the main funding body of WISDOM, the Medical Research Council
(UK), withdrew funding for the study. More recent references discussing trial
outcomes have been added below, and are recommended reading for completion of
this CME activity.
Faculty
affiliations and disclosures are at the end of
this activity.
Release Date: March 31, 2003; Valid for
credit through March 31, 2004
Target Audience
This activity was developed for OB/GYNs and primary care physicians.
Goal
Information on estrogen replacement is rapidly becoming available. At the
same time, practitioners suggest the information is not always clear. This
piece will clarify what is now known about HRT and SERMs, as well as where
the field may be heading.
Learning Objectives
Upon completion of this educational activity, participants should be able
to:
- Create a treatment plan for the prevention of osteoporosis.
- Describe the effects of cardiovascular disease in postmenopausal
women.
- Discuss gynecologic considerations in postmenopausal women.
- Discuss breast cancer and SERMs.
Credits Available
Physicians - up to 2.0 AMA PRA category 1 credit(s)
All other healthcare professionals completing continuing education
credit for this activity will be issued a certificate of participation.
Participants should claim only the number of hours actually spent in
completing the educational activity.
Canadian physicians please note:
Medscape's CME activities are eligible to be submitted for either
Section 2 or Section 4 [when creating a personal learning project] in
the Maintenance of Certification program of the Royal College of
Physicians and Surgeons, Canada [RCPSC]. For details, go to www.mainport.org.
Accreditation Statements
For Physicians

This activity has been planned and implemented in accordance with
Essential Areas and Policies of the Accreditation Council for
Continuing Medical Education (ACCME) through the joint sponsorship of
The Annenberg Center for Health Sciences at Eisenhower and Medscape,
Inc. The Annenberg Center is accredited by the Accreditation Council
for Continuing Medical Education to provide continuing medical
education for physicians.
The Annenberg Center for Health Sciences designates this
educational activity for a maximum of 2.0 hours in category 1 credit
toward the AMA Physician's Recognition Award. Each physician should
claim only those hours of credit that he/she actually spent in the
educational activity.
This activity consists of a website. Successful completion is achieved
by reading the material, reflecting on its implications in your
practice, and completing the assessment component.
The estimated time to complete the activity is 2 hours.

For questions regarding the content of this activity, contact the
accredited provider for this CME/CE activity:
mpederson@annenberg.net.
For technical assistance, contact
CME@webmd.net.
Instructions for Participation and Credit
This online, self-study activity is formatted to include text,
graphics, and may include other multi-media features.
Participation in this self-study activity should be completed in
approximately 2.0 hours. To successfully complete this activity and
receive credit, participants must follow these steps during the period
from March 31, 2003 through March 31, 2004.
- Make sure you have provided your professional degree in your
profile. Your degree is required in order for you to be issued the
appropriate credit. If you haven't, click
here. For information on applicability and acceptance of
continuing education credit for this activity, please consult your
professional licensing board.
- Read the target audience, learning objectives, and author
disclosures.
- Study the educational activity online or printed out.
- Read, complete, and submit answers to the post test questions and
evaluation questions online. Participants must receive a test score
of at least 70%, and respond to all evaluation questions to receive
a certificate.
- When finished, click "submit."
- After submitting the activity evaluation, you may access your
online certificate by selecting "View/Print Certificate"
on the screen. You may print the certificate, but you cannot alter
the certificate. Your credits will be tallied in the CME Tracker.
This activity is supported by an educational grant from Eli Lilly.

Legal Disclaimer
The material presented here does not necessarily reflect the views of Medscape,
The Annenberg Center for Health Sciences, the companies providing educational
grants or the authors and writers. These materials may discuss uses and
dosages for therapeutic products that have not been approved by the United
States Food and Drug Administration. All readers and continuing education
participants should verify all information and consult a qualified healthcare
professional before treating patients or utilizing any therapeutic product
discussed in this educational activity.
Contents of This CME Activity
- The Role of HRT and
SERMs: Evidence-Based Medicine
Introduction
An Ideal Time for Options
Looking Ahead
References
- Osteoporosis:
Fractures and Key Risk Factors
A Global Health Issue
Osteoporosis Redefined
Risk Factors
Morbidity and Mortality
Identifying Risks
Treatment and Fracture Risk Reduction
Ongoing Studies
What's Next?
Conclusion
References
- Cardiovascular
Disease: New Recommendations for Minimizing the Threat
Introduction
Markers of CVD Risk
The Role of HRT in CVD
New HRT Guidelines
Updated Cholesterol Management Guidelines
Use of Statins and SERMs
Conclusion
References
- The Faculty
Speaks: A Roundtable Discussion on Postmenopausal Health Care
Cardiovascular Health
Bone Degeneration
Safety of Postmenopausal Interventions
Conclusion
References
HRT and SERMs: New Guidelines for Patient Management - Part 1
The Role of HRT and SERMs: Evidence-Based Medicine
Thomas E. Nolan, MD, MBA
Introduction
The time for this program has never been better: Researchers are intensely
exploring the role of female hormones in the development of age-related
illnesses such as cardiovascular disease, osteoporosis, and cancer. Such
investigation has unveiled estrogen's benefits in preserving bone mineral
density (BMD) and preventing certain gynecologic cancers, and has provided
evidence that combined estrogen and progesterone exert positive effects on
serum lipid levels and, quite possibly, the risk of coronary artery disease.
This investigation has also unveiled much controversy, which has complicated
prevention and treatment protocols.
As diligent as many healthcare providers have been in communicating to
patients about the various advantages of estrogen replacement therapy (ERT)
and hormone replacement therapy (HRT), many postmenopausal women have been
less enthusiastic about long-term use of these interventions. Although the
many benefits of supplemental estrogen have been documented in numerous
studies, several factors contribute to many patients' lack of interest in and
compliance with ERT and HRT. These include real and perceived breast
tenderness, weight gain, and skin changes, as well as the possible risks of
breast and ovarian cancer.
The Role of HRT and SERMs: Evidence-Based Medicine
Thomas E. Nolan, MD, MBA
An Ideal Time for Options
Because of these limitations on ERT and HRT, a great emphasis has been
placed on identifying estrogen-related therapies that postmenopausal women
might perceive as less fraught with side effects and risks. These efforts have
yielded selective estrogen receptor modulators (SERMs): compounds that act
efficiently through the estrogen receptor but do not increase the amount of
active hormone in the body. The first agent observed to act in this manner was
tamoxifen, used for the prevention of breast cancer in women at high risk for
the disease. Tamoxifen has also been widely observed to exert beneficial
effects on bone,[1] and, more recently, markers for cardiovascular
health.[2] However, tamoxifen has also been shown to incur some
risk of ocular disorders and uterine cancer.[3,4]
Further efforts to improve upon the prevention of age-related disease in
postmenopausal women have led to the development of another SERM, raloxifene,
designed for even higher selectivity in this population. Raloxifene is
currently under investigation for the prevention of breast cancer and may
actually hold promise in several therapeutic areas. Readers should note,
however, that the risk of thromboembolism is the same in women using
raloxifene as in those using exogenous estrogen, and identification of
appropriate disease markers in at-risk patients is warranted. The areas in
which SERMs are being explored for optimum benefit were discussed in a
roundtable meeting entitled "HRT and SERMs: Evidence-Based Data to Guide
Patient Management," the proceeds of which are presented here in the form
of clinical articles and an additional dialogue section titled "The
Faculty Speaks." Brief summaries of the clinical articles appear below.
Skeletal Activity.
Raloxifene decreases bone turnover in a manner similar to that seen with
estrogen replacement. As discussed in the article, "Osteoporosis:
Fractures and Key Risk Factors" by Robin K. Dore, MD, this agent has been
extensively studied for its use in reducing osteoporotic fracture in the
spine, hip, and total body.
Applications in Heart Health.
As cardiovascular disease (CVD) is the number-one killer of women (and
men), healthcare providers are generally enthusiastic about the discovery of
cardiovascular benefits in a new drug. Currently, there is good reason to
believe that the SERMs offer some consistent and positive effects on
low-density lipoprotein cholesterol, without increasing serum triglycerides or
decreasing high-density lipoprotein cholesterol.[2] The article,
"Cardiovascular Disease: New Recommendations for Minimizing the
Threat," by Lori Mosca, MD, MPH, PhD, provides thoughtful insight into
these and other therapies to reduce the incidence of CVD among older women.
The Role of HRT and SERMs: Evidence-Based Medicine
Thomas E. Nolan, MD, MBA
Looking Ahead
SERMs represent a very exciting option for disease prevention in certain
postmenopausal women. In enthusiastic response to our readers, the articles in
this program aim to "cut through the noise" that too often clouds
the issue of postmenopausal hormone-related therapy. It is my hope that you,
the reader, will be able to draw from the insights published here to enhance
the overall well-being of our postmenopausal patient population.
The Role of HRT and SERMs: Evidence-Based Medicine
Thomas E. Nolan, MD, MBA
References
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on
bone mineral density measured by dual energy x-ray absorptiometry in
healthy premenopausal and postmenopausal women. J Clin Oncol.
1996;14(1):78-84.
- Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of
tamoxifen on cardiovascular risk factors in postmenopausal women after 5
years of treatment. J Natl Cancer Inst. 1994; 86:1534-1539.
- Nayfield SG, Gorin MB. Tamoxifen associated eye disease: a review. J
Clin Oncol. 1996;14(3):1018-1026.
- Dessole S, Cherchi PL, Ruiu GA, et al. Uterine metastases from breast
cancer in a patient under tamoxifen therapy: case report. Eur J Gynaecol
Oncol. 1999;20(5-6):416-417.
- Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk
reduction in postmenopausal women treated with raloxifene: 4-year results
from the MORE trial. Breast Cancer Res Treat. 2001;65(2):125-134.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
A Global Health Issue
Osteoporosis is a global public health problem associated with aging, but
it is not an inevitable part of aging. Its diagnosis should be considered in
all women who have had fractures as adults.
The United States, blessed with an aging population, has the dubious
distinction of being a world leader in fractures caused by this disease.[1]
Recognizing that osteoporosis and fractures are largely preventable, the
National Institutes of Health (NIH) convened an NIH Development Consensus
Panel on Osteoporosis Prevention, Diagnosis, and Therapy in March 2000 to
address what it perceived as a "major health threat" affecting 28
million Americans, 80% of them women.[2] (Table 1) Among other
issues, the panel was asked to discuss the meaning of osteoporosis, the
consequences of this disease, and the optimal evaluation and treatment of
osteoporosis and fractures. Given the NIH view that osteoporosis is largely
preventable and frequently not diagnosed, even after a fracture has occurred,
it is not surprising that the panel concluded: "There is a need to
determine the most effective method of educating healthcare professionals and
the public about the prevention, diagnosis, and treatment of
osteoporosis."[2] It is hoped that this article will be a
helpful step in that direction.
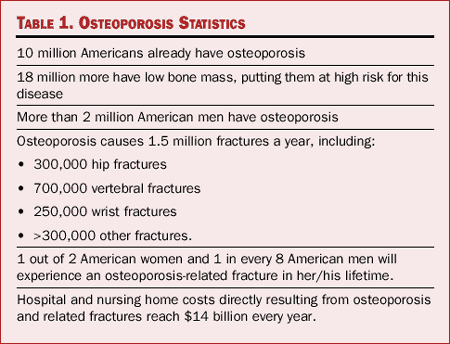
Table 1. Osteoporosis Statistics
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Osteoporosis Redefined
For many years, osteoporosis was viewed simply as a fracture: the outcome
of a "silent" process that was not well understood. While the
complex process leading to an excess of bone resorption over bone formation
remains asymptomatic, major advances have been made in the understanding of
the basic science underlying this process[3,4] and the biomechanics
of fracture.[5]
Osteoporosis is now defined as a skeletal disorder characterized by
compromised bone strength, which predisposes the patient to an increased risk
of fracture.[2] The elements of bone strength are bone density and
bone quality. Bone density, as measured by bone densitometry, is expressed as
grams of mineral per area and the patient's bone density is compared with a
young adult peak bone mass value (called a T-score). Bone quality includes
bone architecture, turnover, damage accumulation (eg, microfractures), and
mineralization. A fracture is the outcome of the application of
failure-inducing force to strength-compromised, or osteoporotic, bone.[2]
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Risk Factors
Osteoporosis can be classified as either primary or secondary. Primary
osteoporosis most often occurs in postmenopausal women and older men, although
it can occur at any age. Secondary osteoporosis is a result of medications,
other conditions, or diseases. Risk factors for primary and secondary
osteoporosis are listed in Table 2.
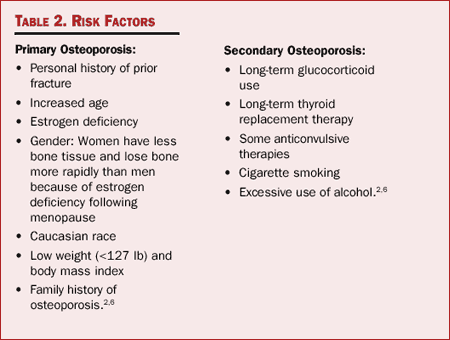
Table 2. Risk Factors
While osteoporosis may be a silent disease, it is striking that the most
important risk factors are apparent from a brief look at a patient and his or
her chart. It is also apparent that ancillary medical personnel, and even the
patient, can perform a rudimentary risk assessment. It is therefore essential
to ensure that the osteoporosis "radar" is on as healthcare
providers go about their daily clinical practice.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Morbidity and Mortality
Osteoporosis and associated fractures are a major public health concern
because of the cost, disability, decreased quality of life, and increased
mortality associated with fractures.
Hip Fractures.
Hip fractures have traditionally been the focus of osteoporosis because
they are easy to diagnose and result in costly hospitalizations and nursing
home placements that are easy to quantify. In addition, there is a 10% to 20%
excess mortality in the 6 months following a hip fracture. For those who
survive, the picture is not encouraging: One year after a hip fracture, 40% of
patients are still unable to walk unassisted, 60% have difficulty with at
least one essential activity of daily living, and 80% are restricted in other
activities, such as driving and shopping for groceries.[7] Nearly
one third of patients with hip fractures enter nursing homes within the year
following a fracture.[2]
Given the morbidity and mortality associated with hip fracture, it is
important to remember that a very effective way to prevent a hip fracture in a
given population may be to utilize hip protectors.[8]
Hip fractures usually occur when women are in their late 70s and 80s.[2]
This is not surprising, because 50% of women in their 80s have osteoporosis
(as measured by bone density studies) at one or both hips, a reflection of the
impact of aging on bones.[9]
Vertebral Fractures.
A key insight of the NIH Consensus Panel was that, while a great deal is
known about the impact of hip fractures, the adverse effects of vertebral
fractures on health, function, and quality of life are often underestimated.
Vertebral fractures lead to chronic pain and disability.[2] They
can result in height loss and kyphosis, with associated postural and height
changes that erode self-esteem. Compression of the thoracic and abdominal
cavities results from multiple vertebral fractures. Multiple thoracic
fractures may result in restrictive lung disease, and lumbar fractures may
alter abdominal anatomy, leading to constipation, abdominal pain, distention,
reduced appetite, and premature satiety.[10-12]
Vertebral fractures are also associated with an increase in mortality. In a
population-based study of survival following osteoporotic fracture, Cooper and
colleagues examined the survival rate of 335 patients who had initial
radiologic diagnoses of vertebral fracture between 1985 and 1989 and compared
them to survival rates of patients who had experienced hip fractures.[13]
They found that, while there was a much greater incidence of mortality among
hip fracture patients within the first 6 months, the estimated survival rates
at 5 years after diagnosis were comparable. Patients with vertebral fractures,
however, had a worse survival rate than expected; that rate diverged steadily
from expected values throughout the course of the study.
Kado and associates found an increased risk of death of 1.23 in patients
who had experienced one or more vertebral fractures.[14] In the
case of severe vertebral fractures, the increased risk of death was 1.34, and
there was an increased risk of death related to respiratory disorders as well.
This study demonstrated that vertebral fractures contribute to comorbid
conditions, such as pneumonia as the result of incomplete lung expansion, and
consequently to mortality.
Cauley and colleagues examined the relative risks of dying following a
clinically related fracture that was apparent to the examining physician, as
reported in the Fracture Intervention Trial.[15] The relative risk
of death following any clinical fracture was 2.15. After a hip fracture, the
risk was 6.68, but after a clinical vertebral fracture, the risk rose to 8.64
-- the highest risk associated with any clinical fractures.
Vertebral fractures are a bearer of bad tidings for the skeleton. They may
reflect generalized loss of bone strength and increase the force on other
vertebral bodies, making them more likely to fail. They may also weaken
muscles, cause pain, and/or upset balance and thereby increase the risk of a
fall. It is not surprising that there is an increased risk of subsequent
fractures following an initial vertebral fracture.
In a study that was conducted to determine whether vertebral fractures
predict subsequent fractures, the authors found that not only do vertebral
fractures indicate an increased risk for subsequent fractures, but that the
greatest risk of subsequent fracture was in the axial skeleton.[16]
After an initial vertebral fracture, the authors observed a 12.6-fold increase
in additional vertebral fractures, a 2.3-fold increase in hip fractures, and a
1.6-fold increase in additional forearm fractures.
A study by Lindsay and associates analyzed data from four large 3-year
osteoporosis treatment trials conducted at 373 study centers in the United
States and abroad.[17] Subjects were a mean age of 74 years, had a
mean of 28 years since menopause, and were known to have had one or more
vertebral fractures. Data analysis showed that patients who had one or more
vertebral fractures at baseline had a 5-fold increased risk of having a
fracture during the first 12 months of the study. Among patients who developed
a vertebral fracture during the study, approximately 20% had a new vertebral
fracture in the next 12 months.
Not every aging woman will develop vertebral fractures, but they are very
common and underdiagnosed. If healthcare providers can prevent a vertebral
fracture, then prevention of the morbidity and mortality associated with that
fracture and the prevention of hip and other fractures may be achieved as
well.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Identifying Risks
The National Institutes of Health Consensus Panel regards the assessment of
bone mass, identification of fracture risk, and determination of who should be
treated as the optimal goals when evaluating patients for osteoporosis.[2]
Until the late 1980s, it was difficult to identify the patients who were at
risk for a fracture before it occurred. Since that time, bone mineral density
(BMD) measurements have become fairly common. While BMD is not a true
measurement of bone strength, it is the best surrogate measurement available
at present.
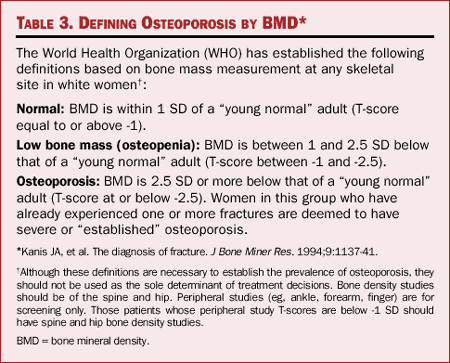
Table 3. Defining Osteoporosis by BMD
The World Health Organization (WHO) operationally defines osteoporosis as
bone density 2.5 SD below the mean for young white adult women (Table 3).
Clinicians now use risk factors to help determine which patients should be
evaluated for osteoporosis with a bone density test. Several important risk
factors for osteoporosis have been identified; however, the list can be
expanded to include the following:
- Adults with vertebral, rib, hip, or distal forearm fractures should be
evaluated for the presence of osteoporosis. The National Osteoporosis
Foundation (NOF) recommends that any woman who has had a fracture as an
adult be evaluated for osteoporosis.[18]
- Since osteoporosis increases with age, the NOF recommends that every
woman aged 65 years and older have a bone density test.[18]
- Corticosteroid (glucocorticoid) use is the major cause of secondary
osteoporosis.
- Estrogen deficiency leads to osteoporosis, particularly in women who
experience rapid bone loss following menopause or who have early menopause
(ie, before age 40). Women with irregular periods prior to menopause may
have osteoporosis due to estrogen deficiency. Also, female athletes who
don't have regular menstrual periods may have osteoporosis at an early
age, particularly if this behavior is accompanied by an eating disorder.
The NOF recommends that every woman aged 65 years and older have a bone
density test.
- Family history is very important, and should include both the mother's
and the father's side of the family. The genetic tendency to develop
osteoporosis is not exclusive to the female's side of the family, nor is
it exclusively a female tendency. Patients who are diagnosed with
osteoporosis should be encouraged to talk to their brothers and sons about
osteoporosis screening and treatment.
- Older patients who lose a significant amount of body weight (ie, 5%) are
at risk for bone mass loss as well.[19]
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Treatment and Fracture Risk Reduction
A patient who has been diagnosed with osteoporosis should be treated
promptly and aggressively. The NOF recommends initiating therapy to reduce
fracture risk in women with BMD T-scores below -2.0 SD in the absence of risk
factors and in women with T-scores below -1.5 SD if other risk factors are
present. Women aged older than 70 years and who have multiple risk factors
(especially those with previous fractures) are at enough of a risk for
fracture to begin treatment without BMD testing.[18]
Several options for prevention or treatment of osteoporosis are available,
including therapies that enhance bone mass and reduce risk of fractures. All
current options are antiresorptive therapies: They inhibit bone resorption by
osteoclasts.
Hormone Replacement.
Hormone replacement therapy (HRT) is indicated for the prevention of
osteoporosis. Several short-term studies and a few longer-term studies using
BMD as the primary outcome have shown significant efficacy. For example, the
Postmenopausal Estrogen/ Progestin Interventions (PEPI) trial showed that HRT
increases bone density in the spine and the hip and produces a reduction in
bone turnover.[20] There have not, however, been any large,
randomized, controlled clinical trials showing fracture reduction with HRT.
The Women's Health Initiative (initiated in 1992, with a planned completion
date of 2007[21]) may show that HRT prevents fractures, but at the
present time, no data are available. As HRT may be associated with a modest
increase in the risk of breast cancer with long-term use and is associated
with increased risk of deep-vein thrombosis (DVT), the clinician is advised to
seek alternative treatments for women diagnosed with or at risk for
osteoporosis who also have a history of, or at significant risk for, breast
cancer or DVT. HRT may have significant side effects in some individuals,
including vaginal bleeding, breast tenderness, mood disturbances,[18]
and gallbladder disease.[17]
SERMs.
The NIH Consensus Panel considered the development of selective estrogen
receptor modulators (SERMs) "an important new thrust in osteoporosis
research."[2] The interest in developing a SERM for the
prevention and treatment of osteoporosis was driven by the observation that
tamoxifen, an effective adjuvant therapy for breast cancer, has estrogen-like
effects on bone.[22]
The SERM raloxifene, approved for the prevention of postmenopausal
osteoporosis in 1997 and the treatment of postmenopausal osteoporosis in 1999,
has estrogen-like effects on bone, but acts as an estrogen antagonist on
breast and endometrial tissue.[23]
Raloxifene has been shown to prevent bone loss at the spine, hip, and total
body.[23] More importantly, the 3-year randomized Multiple Outcomes
of Raloxifene Evaluation (MORE) trial demonstrated that raloxifene
significantly reduced the risks of vertebral fracture. At 1 year, therapy with
raloxifene resulted in a 68% reduction in clinical vertebral fractures. At 3
years, raloxifene decreased vertebral fractures in women who had not had
fractures at baseline by 55%. In women who had vertebral fractures entering
the trial, raloxifene produced a 30% reduction.[24] The MORE trial
was continued into a fourth year, but because the data had not been analyzed
and the significance of the reduction in vertebral fractures was not known at
that point, women who continued the trial into the fourth year were allowed to
add a bone-active agent, with the exception of estrogen. The investigators and
participants remained blinded as to whether patients were taking raloxifene or
placebo. At 4 years, raloxifene continued to provide fracture protection for a
significant number of women. Among women who had no fractures at the start of
the study 4 years earlier, raloxifene produced a 49% reduction in vertebral
fractures. In the cohort with existing vertebral fractures at study entry,
there was a 34% reduction.[25]
Raloxifene increases the risk of DVT to a degree similar to that observed
with estrogen. In addition, an increase in hot flashes is observed
(approximately 6% more than with placebo). Thus, raloxifene should not be used
to treat menopausal symptoms.[18] However, for asymptomatic women
who were taking estrogen for menopausal symptoms and wish to remain on
preventive therapy, it is reasonable to change their therapy to raloxifene.
Calcitonin.
Salmon calcitonin is a synthetic hormone that inhibits bone resorption and
is approved for the treatment of osteoporosis in women 5 years after menopause
has occurred. It is available as an intranasal spray; a single daily dose
provides 200 units (u) of the drug. In a 5-year, double-blind, randomized,
placebo-controlled trial of 511 postmenopausal women with established
osteoporosis, participants were randomized to receive 100, 200, or 400 u of
intranasal calcitonin daily.[26] Results showed that only the 200-u
dose produced a significant (36%) reduction of vertebral fractures, with no
reduction in hip fractures and no significant change in either bone density or
bone turnover.
Bisphosphonates.
Alendronate sodium was the first bisphosphonate approved for use in
osteoporosis. Alendronate 5 mg daily and 35 mg once weekly are approved by the
US Food and Drug Administration for the prevention of osteoporosis. The dose
for the treatment indication is 10 mg daily or 70 mg once weekly. Alendronate
is also approved for the treatment of bone loss in both men and women as a
result of prolonged glucocorticoid use and for the treatment of male
osteoporosis.[18] Large, randomized, controlled trials have shown
that alendronate reduces the incidence of fracture at the spine, hip, and
wrist by 50% in patients with osteoporosis.[27]
Alendronate must be taken on an empty stomach, first thing in the morning,
at least 30 minutes before having any food or beverage. It must be taken with
a large glass of water, and patients are advised to sit upright or stand for
30 minutes after administration. In clinical trials, the incidence of side
effects with alendronate was no different than placebo; however, in clinical
experience, upper gastrointestinal disturbance, particularly esophageal
symptoms (eg, chest pain, heartburn, painful or difficult swallowing) has been
a problem with alendronate in some patients. A rare (probably <1%) reported
complication of alendronate is esophageal ulceration.[18]
Risedronate is a second bisphosphonate approved for use in osteoporosis.
Data from the Vertebral Efficacy with Risedronate trial showed a 41% reduction
in new x-ray-apparent vertebral fractures, and a 39% reduction in nonvertebral
fractures.[28] Risedronate also demonstrated a significant
reduction in hip fracture in a population of postmenopausal women aged 70 to
79 years.[29] Risedronate is also approved for the prevention and
treatment of glucocorticoid-induced osteoporosis in women and men. Risedronate
must be taken using the same regimen as alendronate: on an empty stomach with
a full glass of water, remaining upright, and waiting 30 minutes before having
any food or beverage.
Combination Therapy.
Studies have shown that increases in bone density with combination therapy
are greater than those achieved by either agent alone, but without additive
increases. Because there are no data regarding fracture reduction with
combination therapy, and no bone biopsy data, many bone specialists are
hesitant to recommend combination therapies until the data show greater
fracture reduction. However, if a patient who takes estrogen for menopausal
symptoms needs treatment for osteoporosis, most bone specialists will continue
her on estrogen therapy and add a bisphosphonate. It is currently not
advisable that estrogens and raloxifene be taken together; investigation is
underway.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Ongoing Studies
Risedronate is currently being evaluated to determine whether a once-weekly
dose is as effective as the 5-mg daily dose presently approved. A study of
nasal calcitonin is ongoing to determine the mechanism by which calcitonin
prevents fractures. A 5-year trial comparing alendronate with raloxifene
initiated enrollment in the fall of 2001. This is a very large study, with
primary endpoints including vertebral and nonvertebral fractures that will
give clinicians the first "head-to-head" comparison of the
antifracture efficacy and safety of a SERM and a bisphosphonate. This trial
may provide answers as to why, when compared to bisphosphonates in
postmenopausal women, raloxifene has produced similar vertebral fracture
reductions with small increases in BMD and small reductions in bone turnover.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
What's Next?
In the near future, we hope to have anabolic therapies that will actually
increase bone formation. The first of these, parathyroid hormone, which may be
on the market within the year, demonstrated significant reduction in vertebral
fractures.[30] [Teriparatide therapy for osteoporosis was approved
by the FDA at the end of November 2002. The teriparatide package insert
includes a "black box" warning, acknowledging an increased risk of
osteosarcoma in rats treated with relatively high doses of teriparatide.
Treatment beyond 2 years is not currently recommended.] New SERMs are under
development, as are oral formulations of salmon calcitonin. Intravenous
bisphosphonates are also being evaluated for the treatment of osteoporosis.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
Conclusion
For now, our most effective means of treating osteoporosis remains
identifying patients, starting them on antiresorptive therapy, if appropriate,
and sending-them out into the world as "ambassadors for bone
health." Healthcare providers must also remember the basics: counseling
all patients to take adequate calcium and vitamin D, reviewing and encouraging
appropriate exercise activities, and discussing fall prevention. In addition,
clinicians must be mindful that hip protectors may be the most effective way
to prevent hip fractures in a given population.
Many postmenopausal women with existing osteoporotic fractures are not
diagnosed and treated. Only 22.9% of women with Colles fractures were treated
for osteoporosis in a large retrospective study of more than 3 million
patients in 30 states.[31]
Excellent therapies are available now, and it is hoped that better ones
will be available in the near future. With bone density studies, clinicians
have the tool to diagnose osteoporosis. By reviewing patients' charts, looking
for previous fracture, identifying a positive family history of the disease
and medications that could contribute to it, and examining patients for height
loss and kyphosis and other risk factors, clinicians can diagnose and treat
patients before a fracture occurs.
Osteoporosis: Fractures and Key Risk Factors
Robin K. Dore, MD
References
- Osteoporosis: both health organizations and individuals must act now to
avoid an impending epidemic. Press Release WHO/58. October 11, 1999.
Available at http://www.who.int/infpr-1999/en/pr99-58.html.
Accessed January 4, 2002.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis
and Therapy. JAMA. 2001;285:785-795. Available at http://consensus.nih.gov/cons/111/111_statement.htm#introduction.
Accessed January 4, 2002.
- Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated
fibroblast under central surveillance. Science. 2000;289:1501-1504.
- Teitelbaum S. Bone resorption by osteoclasts. Science. 2000;289:
1504-1508.
- Van Der Linden JC, Verhaar JA, Weinans H. Mechanical consequences of
bone loss in cancellous bone. J Bone Miner Res. 2001;16: 457-465.
- Osteoporosis Overview. Fact Sheets. National Institutes of Health
Osteoporosis and Related Bone Diseases, National Resource Center.
Available at http://www.osteo.org/docs/30.464633275.html.
Accessed January 4, 2002.
- Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in
elderly people with use of a hip protector. N Engl J Med.
2000;343:1506-1513.
- Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to
severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68-71.
- Melton LJ. How many women have osteoporosis now? J Bone Miner Res.
1995;10:175-177.
- Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in
patients with spinal osteoporotic fractures. Osteoporos Int.
1998;8(3):261-267.
- Silverman SL. The clinical consequences of vertebral compression
fracture. Bone. 1992;13 Suppl (2): S27-S31.
- Cooper C. The crippling consequences of fractures and their impact on
quality of life. Am J Med 1997; 103(2A):12S-17S.
- Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of
survival after osteoporotic fractures. Am J Epidemiol.
1993;137(9):1001-1005.
- Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality
in older women: a prospective study. Study of Osteoporotic Fractures
Research Group. Arch Int Med. 1999;159:1215-1229.
- Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following
clinical fracture. Osteoporos Int. 2000;11:556-561.
- Melton J. Vertebral fractures predict subsequent fractures. Osteoporos
Int. 1999;213-221.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture
in the year following a fracture. JAMA. 2001;285(3):320-323.
- National Osteoporosis Foundation. Physician's Guide to Prevention and
Treatment of Osteoporosis, 1998. Available at: http://www.nof.org.
physguide.htm. Accessed January 11, 2002.
- Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for
longitudinal bone loss in elderly men and women: the Framingham
Osteoporosis Study. J Bone Miner Res. 2000;15:710-720.
- The Writing Group for the PEPI. Effects of hormone therapy on bone
mineral density: results from the postmenopausal estrogen/progestin
interventions (PEPI) trial. JAMA. 1996;276(17):1389-1396.
- The Women's Health Initiative Study Group. Design of the Women's Health
Initiative clinical trial and observational study. Control Clin Trials.
1998;19(1):61-109.
- Fontana A, Delmas PD. Clinical use of selective estrogen receptor
modulators. Curr Opin Rheumatol. 2001; 13(4):303-309.
- Johnston CC, Bjarnason NH, Cohen FJ, et al. Long-term effects of
raloxifene on bone mineral density, bone turnover, and serum lipid levels
in early postmenopausal women: three-year data from 2 double-blind,
randomized, placebo-controlled trials. Arch Intern Med.
2000;160(22):3444-3450.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture
risk in postmenopausal women with osteoporosis treated with raloxifene:
results from a 3-year randomized clinical trial. Multiple outcomes of
raloxifene evaluation (MORE) investigators. JAMA. 1999;282(7):637-645.
- Eastell R, Mallinak N, Weiss S, et al. Biological variability of serum
and urinary N-telopeptides of type I collagen in postmenopausal women. J
Bone Miner Res. 2000;15(1):522-529.
- Chestnut CH, Silverman S, Andriano K, et al. A randomized trial of nasal
spray salmon calcitonin in postmenopausal women with established
osteoporosis: the prevent recurrence of osteoporotic fractures study.
PROOF Study Group. Am J Med. 2000;109(4):267-276.
- Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with
alendronate in women with osteoporosis: the Fracture Intervention Trial.
FIT Research Group. J Clin Endocrinol Metab. 2000;85 (11):4118-4124.
- Harris ST, Watts, NJ, Genant HK, et al. Effects of risedronate treatment
on vertebral and on vertebral factures in women with postmenopausal
osteoporosis: a randomized controlled trial. Vertebral Efficacy with
Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1244-1252.
- McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the
risk of hip fracture in elderly women: Hip Intervention Program Study
Group. N Engl J Med. 2001;344 (5):333-340.
- Neer R, Arnaud CD, Zanchetta JR, et al. Recombinant human PTH (rhPTH
1-34] reduces the risk of spine and non-spine fractures in women with
postmenopausal osteoporosis. N Engl J Med. 2001; 344(19):1434-1441.
- Freedman KB, Kaplan FS, Bilker WB, et al. Treatment of osteoporosis: are
physicians missing an opportunity? J Bone Joint Surg Am.
2000;82A(8):1063-1070.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
Introduction
The successful assessment and management of woman's cardiovascular health
after menopause is reliant on several factors.
Clinicians treating postmenopausal patients must be well versed on an
increasing number of cardiovascular disease (CVD) markers and risk factors.
The benefits and risks of hormone replacement therapy (HRT) must be weighed
with extreme care; familiarity with emerging therapies and new indications is
crucial for the optimal treatment of CVD in postmenopausal women.
This program outlines the important markers of CVD, with attention to
several new markers that likely are not familiar to most primary care
physicians and OB/GYNs. The role of HRT in cardioprotection is addressed,
based on findings from three major clinical trials of HRT in CVD.
Additionally, new recommendations recently issued by the American Heart
Association (AHA) concerning HRT use in CVD are summarized, as well as updated
guidelines for cholesterol testing and management from the third Adult
Treatment Panel (ATP III) of the National Cholesterol Education Program (NCEP).
Finally, the roles of statins and selective estrogen receptor modulators (SERMs)
in treating and preventing CVD are reviewed.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
Markers of CVD Risk
Traditional.
The traditional markers of CVD risk in postmenopausal women include
elevated levels of low-density lipoproteins (LDL), low levels of high-density
lipoproteins (HDL), high triglycerides, family history of heart disease,
diabetes, hypertension, obesity, physical inactivity, and smoking. These
markers for CVD have been well established over many years and remain the most
important markers to emphasize to patients.
Novel.
In recent years, several new markers have been found that may prove
critical in the assessment of CVD risk. While not commonly used by the primary
care physician or OB/GYN, these markers are often used by cardiologists to
assess CVD risk in women who appear to be at high risk or have already
experienced a CVD-related event.
C-reactive protein: As a marker for systemic inflammation, C-reactive
protein (CRP) is considered an important component in determining the risk for
CVD. Researchers have known that CRP levels are higher in people with heart
disease, and they have long speculated that atherosclerosis is an inflammatory
process. With a simple but highly sensitive blood test that measures CRP
levels, the extent of underlying atherosclerosis can be determined and the
risk of future heart attack and stroke predicted. In a 3-year study of more
than 28,000 healthy postmenopausal women, CRP was the strongest single
predictor of future cardiac events, such as heart attacks.[1] Women
with the highest levels of CRP had a fivefold increase in the risk of
developing CVD and a sevenfold increase in the risk of having a heart attack
or stroke, compared with those who had the lowest levels of CRP. Levels of CRP
predicted these events even among apparently low-risk women, such as those who
did not smoke, had no evidence of high cholesterol, and had no family history
of heart disease. The investigators cautioned that standard laboratory tests
are not sufficient for determining CVD risk. Only high-sensitivity or
ultrasensitive tests for CRP are capable of predicting CVD risk.
Homocysteine: Elevated levels of this amino acid have been correlated with
artery damage, blood clotting, myocardial infarction, stroke, and other
manifestations of CVD. Inadequate intake of folic acid and B6 and B12 vitamins
is thought to cause increases in homocysteine. Plasma homocysteine levels
increase dramatically when a woman reaches menopause, and this is thought to
play a role in the increased incidence of vascular disease, cancer, and
possibly osteoporosis in postmenopausal women.[2,3] However, the
strength of the association between homocysteine and CVD is not certain, and
controversy exists about whether lowering homocysteine levels will reduce
Coronary Heart Disease (CHD) risk.
Fibrinogen: The body depends on this protein for proper blood clotting.
However, high levels of fibrinogen can restrict blood flow and lead to
hardened arteries and accumulation of plaque. Increased production of
fibrinogen is associated with traditional CVD risk factors (eg, obesity,
smoking, physical inactivity, diabetes).[4] Aging also has been
linked to high fibrinogen levels.[5] In healthy volunteers,
fibrinogen levels were markedly higher in those aged more than 50 years
compared with younger participants. Moreover, higher fibrinogen levels in
older adults corresponded with a 60% drop in dilation ability, whereas other
markers, such as HDL, LDL, and total cholesterol did not significantly
correlate with age or dilation ability. As such, the investigators noted that
fibrinogen is a highly predictive marker for reduced elasticity of the
endothelium.
Lipoprotein(a): Lipoprotein(a) [Lp(a)] is composed of an LDL-like particle
and apolipoprotein A. An elevated blood concentration of Lp(a) is associated
with an increased risk of atherosclerosis and coronary artery disease.[6]
However, Lp(a) is not considered as great a risk for CHD as are the
traditional risk factors. Moreover, measurement of Lp(a) is not standardized,
nor is it widely available in clinical practice. It has been suggested that
measurement of Lp(a) be reserved for those with a strong family history of
early CHD or with genetic predisposition to hypercholesterolemia, such as
familial hypercholesterolemia.[7,8]
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
The Role of HRT in CVD
Although HRT has an established role in the prevention of osteoporosis and
relief of vasomotor symptoms, controversy remains regarding the role of HRT in
CVD. Data from observational studies largely support the use of HRT in
postmenopausal women for a reduced risk of CVD; however, recent randomized,
controlled clinical trials have found no overall benefit of HRT in CVD risk
reduction or prevention.
The Heart and Estrogen/progestin Replacement Study (HERS) was the first
large-scale randomized trial of HRT for prevention of coronary heart disease (CHD)
in postmenopausal women.[9] After an average of 4.1 years of
follow-up, there was no overall benefit of HRT on secondary prevention of CHD.
In fact, during the first year of treatment, a significant (52%) increase in
cardiovascular events was noted in the HRT group compared with placebo. Fewer
events occurred as the study progressed, and benefit with HRT was seen by year
4. Based on these findings, the investigators recommended that HRT not be
started for secondary prevention of CHD. A 3-year follow-up study, HERS-II, is
currently underway to evaluate the longer term effects of HRT in secondary
prevention of CHD.[10]
In the Estrogen Replacement and Atherosclerosis (ERA) trial, no benefit was
seen after 3 years of treatment with HRT or with estrogen alone on the
progression of atherosclerosis in postmenopausal women with established
disease.[11] These results support those of HERS and consequently
lend more support to the recommendation that HRT not be initiated for
secondary prevention of CHD.
Several ongoing prospective angiographic trials should offer more insight
into the effect of HRT on the progression of coronary disease. Angiographic
endpoint trials include the Estrogen and Bypass Graft Atherosclerosis
Regression trial (EAGER), the Women's Lipid Lowering Heart Atherosclerosis
Trial (WELLHART), and the Women's Atherosclerosis Vitamin/Estrogen trial
(WAVE). In primary prevention, trials include the Women's Health Initiative (WHI;
of which the combination HRT arm has been discontinued as of July 2002) and
the Women's International Study of Long-Duration Oestrogen after Menopause
(WISDOM). In secondary prevention, trials include the Heart and Estrogen/progestin
Replacement Study follow-up (HERS II) and the Estrogen in the Prevention of
Reinfarction Trial (ESPRIT).[10]
One of these ongoing trials is the Women's Health Initiative (WHI), which
includes a large-scale randomized trial to assess the effects of HRT on
primary prevention of CHD over an 8- to 12-year period.[12] The
latest update from the WHI indicates an early increased risk of CVD events
among healthy women randomized to estrogen alone or HRT compared with placebo.
Fewer than 1% of women taking estrogen only or HRT experienced early CVD
events. Future updates are awaited for further information regarding the
long-term effects of estrogen or HRT on primary prevention of CVD.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
New HRT Guidelines
An extensive analysis of the evidence from these current and ongoing
clinical trials led to the development of new guidelines by the AHA for the
role of HRT in CVD.[10] The new guidelines consider the use of HRT
in both secondary and primary prevention. In summary, the AHA recommends that
HRT not be initiated for the purpose of secondary prevention. This is based on
the lack of definitive evidence that HRT will prevent coronary events,
particularly among women with established coronary disease. For primary
prevention, the AHA states that decisive clinical recommendations for HRT use
must await the results of ongoing randomized clinical trials. However,
possible coronary benefits and risks can be factored into the treatment
decision for primary prevention.
The AHA emphasizes that the driving factor of whether HRT should be used in
primary prevention should be based mainly on established noncoronary benefits,
such as osteoporosis prevention, relief of vasomotor symptoms, and patient
preference. The decision to use HRT in secondary prevention should be driven
by noncoronary factors as well. For women who have an acute coronary event or
are immobile, the AHA suggests discontinuation of HRT.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
Updated Cholesterol Management Guidelines
Clinicians should also be familiar with the Third Report of the NCEP Expert
Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in
Adults (ATP III).[13] This report, issued in 2001, describes in
great depth the NCEP updated clinical guidelines for cholesterol testing and
management. These guidelines are outlined in the table below.
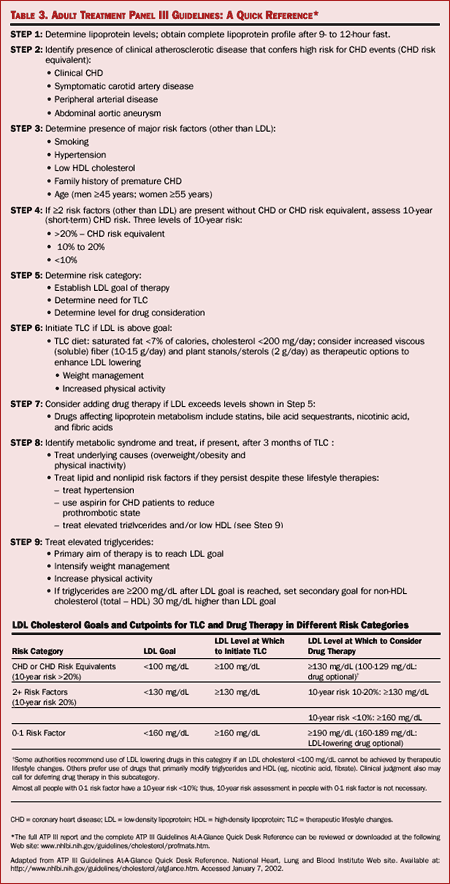
Table. (click
to enlarge) Adult Treatment Panel III Guidelines: A Quick Reference
The updated guidelines share many features with previous ATP
recommendations for the management of CVD in women with hypercholesterolemia,
but emphasize more aggressive lowering of elevated LDL levels. For example,
the ATP III guidelines recommend a target LDL of less than 100 in women who
have greater than a 20% 10-year risk for CHD. This includes women with
peripheral vascular disease, coronary artery disease, abdominal aortic
aneurysms, or diabetes.
Another advancement in the ATP III guidelines is the classification of the
metabolic syndrome, a very common problem in postmenopausal women. By
definition, metabolic syndrome can be diagnosed in women with at least three
of the following factors: a waist circumference greater than 35 inches,
triglycerides at least 150 mg/dL, HDL cholesterol lower than 50 mg/dL, blood
pressure at least 130/85 mm Hg, or a fasting glucose at least 110 mg/dL.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
Use of Statins and SERMs
Statins -- First-line therapy for CVD: Based on the AHA recommendations and
the ATP III guidelines, the general consensus for CVD prevention and
management in postmenopausal women is modification of lifestyle supplemented
with pharmacotherapy when indicated. Statins are currently considered the most
effective therapy for lowering LDL cholesterol levels.[14, 15]
Statins block the synthesis of cholesterol in the liver, which effects a
decrease in plasma LDL levels. These agents have been shown to dramatically
reduce levels of total serum cholesterol, thus demonstrating their beneficial
effect in reducing CHD events.[14] Studies among postmenopausal
women with hypercholesterolemia have reported that statins reduced LDL levels
by 25.4% to 45%.[15-20] Statins are also more effective than ERT/HRT
in reducing LDL levels,[16, 17, 19, 20] and have demonstrated
significant triglyceride-lowering effects compared with HRT.[20]
Thus, for women with elevated LDL cholesterol, statins are now considered
first-line therapy instead of ERT/HRT.
SERMs -- Emerging cardioprotective effects: SERMs are drugs with mixed
agonist/antagonist action on estrogen receptors in different tissues.
Raloxifene is a SERM indicated for both the prevention and treatment of
osteoporosis. Tamoxifen also belongs to the SERM class of drugs, and has been
shown to reduce the incidence of breast cancer in healthy women at high risk
of developing the disease. Studies of SERMs in CHD have shown that they are
capable of reducing LDL levels without affecting HDL or triglyceride levels
significantly in postmenopausal women.[21-25]
Studies in postmenopausal women also have demonstrated beneficial effects
of SERMs on several new markers of CVD. Both tamoxifen and raloxifene have
been shown to decrease levels of Lp(a),[23, 26] with tamoxifen
having a greater effect than that seen with conjugated equine estrogen.[26]
SERMs also have a greater ability to lower fibrinogen levels compared with HRT.[23,
27] In a recent study of tamoxifen, fibrinogen levels were reduced by
22%, and the drug was effective in reducing levels of CRP by 26%.[28]
These trials offer encouraging evidence for a potential role of SERMs in
CVD prevention; however, further research is needed to confirm the clinical
relevance of the beneficial effect on many markers of CVD. The Raloxifene Use
for the Heart (RUTH) Study is underway to examine more than 10,000
perimenopausal women for CVD and breast cancer.[29] Future
decisions regarding SERM use will likely be influenced by the effects of
compounds on more than one organ system.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
Conclusion
Because CVD is the leading cause of death and an important cause of
disability in postmenopausal women, it is imperative that clinicians are
familiar with its emerging markers. It is also important that clinicians
understand all of the agents available to help women achieve lipid targets and
optimize risk-reducing strategies.
Cardiovascular Disease: New Recommendations for Minimizing the Threat
Lori Mosca, MD, MPH, PhD
References
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and
other markers of inflammation in the prediction of cardiovascular disease
in women. New Engl J Med. 2000;342: 836-843.
- Anker G, Lonning PE, Ueland PM, et al. Plasma levels of the atherogenic
amino acid homocysteine in postmenopausal women with breast cancer treated
with tamoxifen. Int J Cancer. 1995;60:365-368.
- Boers GH, Smals AG, Trijbels FJ, et al. Unique efficiency of methionine
metabolism in premenopausal women may protect against vascular disease in
the reproductive years. J Clin Invest. 1983;72:1971-1976.
- Stec JJ, Silbershatz H, Tofler GH, et al. Association of fibrinogen with
cardiovascular risk factors and cardiovascular disease in the Framingham
Offspring Population. Circulation. 2000; 102:1634-1638.
- Allen JD, Wilson JB, Tulley RT, et al. Influence of age and normal
plasma fibrinogen levels on flow-mediated dilation in healthy adults. Am J
Cardiol. 2000; 86:703-705.
- Zenker G, Koltringer P, Bone G, et al. Lipoprotein(a) as a strong
indicator for cerebrovascular disease. Stroke. 1986;17:942-945.
- Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for
coronary artery disease. Am J Cardiol. 1998;82:57U-66U; discussion 86U.
- Marcovina SM, Hegele RA, Koschinsky ML. Lipoprotein(a) and coronary
heart disease risk. Curr Cardiol Rep. 1999;1:105-111.
- Hulley S, Grady D, Bush T, et al, for the Heart and Estrogen/progestin
Replacement Study (HERS) Research Group. Randomized trial of estrogen plus
progestin for secondary prevention of coronary heart disease in
postmenopausal women. JAMA. 1998; 280:605-613.
- Mosca L, Collins R, Herrington DM, et al. Hormone replacement therapy
and cardiovascular disease: a statement for healthcare professionals from
the American Heart Association. Circulation. 2001;104:499-503.
- Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen
replacement on the progression of coronary-artery atherosclerosis. N Engl
J Med. 2000;343:522-529.
- The Women's Health Initiative Study Group. Design of the Women's Health
Initiative clinical trial and observational study. Control Clin Trials.
1998;19:61-109.
- National Cholesterol Education Program. Third Report of the Expert Panel
on Detection, Evaluation, and Treatment of High Blood Cholesterol in
Adults (Adult Treatment Panel III). National Heart, Lung and Blood
Institute Web site. Available at http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm.
Accessed January 7, 2002.
- Gould AL, Rossouw JE, Santanello NC, et al. Cholesterol reduction yields
clinical benefit: impact of statin trials. Circulation. 1998;97:946-952.
- Gotto AM, Jr. Cholesterol management in theory and practice.
Circulation. 1997;96:4424-4430.
- Davidson MH, Testolin LM, Maki KC, et al. A comparison of estrogen
replacement, pravastatin, and combined treatment for the management of
hypercholesterolemia in postmenopausal women. Arch Intern Med.
1997;157:1186-1192.
- Herrington DM, Werbel BL, Riley WA, et al. Individual and combined
effects of estrogen/progestin therapy and lovastatin on lipids and
flow-mediated vasodilation in postmenopausal women with coronary artery
disease. J Am Coll Cardiol. 1999;33:2030-2037.
- Ushiroyama T, Ikeda A, Ueki M, Sugimoto O. Efficacy and short-term
effects of pravastatin, a potent inhibitor of HMG-Co A reductase, on
hypercholesterolemia in climacteric women. J Med. 1994;25:319-331.
- Darling GM, Johns JA, McCloud PI, Davis SR. Estrogen and progestin
compared with simvastatin for hypercholesterolemia in postmenopausal
women. N Engl J Med. 1997;337:595-601.
- Sbarouni E, Kyriakides ZS, Kremastinos DT. The effect of hormone
replacement therapy alone and in combination with simvastatin on plasma
lipids of hypercholesterolemic postmenopausal women with coronary artery
disease. J Am Coll Cardiol. 1998;32:1244-1250.
- Love RR, Wiebe DA, Newcomb PA, et al. Effects of tamoxifen on
cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991;
115:860-864.
- Love RR, Wiebe DA, Feyzi JM, et al. Effects of tamoxifen on
cardiovascular risk factors in postmenopausal women after 5 years of
treatment. J Natl Cancer Inst. 1994;86:1534-1539.
- Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum
lipids and coagulation factors in healthy postmenopausal women. JAMA.
1998; 279:1445-1451.
- Draper MW, Flowers DE, Huster WJ, et al. A controlled trial of
raloxifene (LY139481) HCl: impact on bone turnover and serum lipid profile
in healthy postmenopausal women. J Bone Miner Res. 1996;11:835-842.
- Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone
mineral density, serum cholesterol concentrations, and uterine endometrium
in postmenopausal women. N Engl J Med. 1997;337:1641-1647.
- Shewmon DA, Stock JL, Rosen CJ, et al. Tamoxifen and estrogen lower
circulating lipoprotein(a) concentrations in healthy postmenopausal women.
Arterioscler Thromb. 1994;14:1586-1593.
- Grey AB, Stapleton JP, Evans MC, et al. The effect of the antiestrogen
tamoxifen on bone mineral density in normal late postmenopausal women. Am
J Med. 1995;99:636-641.
- Cushman M, Costantino JP, Tracy RP, et al. Tamoxifen and cardiac risk
factors in healthy women: suggestion of an anti-inflammatory effect.
Arterioscler Thromb Vasc Biol. 2001;21:255-261.
- Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the
Raloxifene Use for The Heart (RUTH) study. Am J Cardiol. 2001;88:392-395.
The Faculty Speaks: A Roundtable Discussion on Postmenopausal Health Care
Thomas E. Nolan, MD, MBA; Susan Johnson, MD; Robin Dore, MD; Jane Cauley,
DrPH; Lori Mosca, MD, MPH, PhD
Cardiovascular Health
The following are excerpts from a closed roundtable discussion that took
place in October, 2001, entitled "HRT and SERMs: Evidence-Based Data to
Guide Patient Management." Dr Thomas E. Nolan acted as moderator.
As noted in "Cardiovascular Disease: New Recommendations for
Minimizing the Threat," by Lori Mosca, MD, MPH, PhD, a great need exists
for education, screening, and intervention for cardiovascular illness in
postmenopausal patients.
Dr Nolan: When it comes to the prevention and management of cardiovascular
disease (CVD), for many years we've segregated male and female patients.
However, data are beginning to show that women have some of the same problems
with coronary arteries as men do. Also, many OB/GYNs are not well attuned to
the difference between primary and secondary prevention; in many cases, by the
time secondary prevention is needed, they have already lost control of that
patient.
Dr Johnson: What should be the approach in treating women who don't have
proven coronary heart disease but are at very high risk, because of such
conditions as diabetes and hyperlipidemia? Should we be giving them estrogen?
Dr Mosca: The question you are raising is one of risk equivalence. The
American Heart Association guidelines do not recommend estrogen for secondary
prevention. For nearly all postmenopausal women, there's some degree of
atherosclerosis. At some point, when it becomes clinically evident, we call
its management "secondary prevention." The problem with that way of
thinking is that the first time a woman manifests coronary heart disease, two
thirds of the time it's with sudden death. There are no prior symptoms. The
OB/GYN is in a unique position to treat women more aggressively and to not
wait until there is a diagnosis.
Even in women who are 55 or recently postmenopausal and have one or more
risk factors, I am aggressive in the prevention of CVD.
Dr Nolan: I try to intervene with patients early in menopause as well. I'm
not always successful, because patients are often not yet willing to accept
menopause-related interventions.
Pharmacotherapy.
Dr Mosca: As for hormone replacement therapy (HRT), it's generally not
being used now as a mechanism for the prevention of heart disease. In the
cardiovascular community, the data appear to be insufficient to use it when
there are many other methods [eg, physical activity, smoking cessation,
nutrition, lipid lowering via statin therapy, blood pressure medication] of
controlling CVD.
Dr Dore: What about primary versus secondary prevention and low dose
aspirin in women?
Dr Mosca: For secondary prevention, aspirin is well accepted. For primary
prevention, there's an ongoing trial right now. We do not generally recommend
aspirin in the setting of primary prevention.
Even though a benefit has been seen on an epidemiological level, mainly
among women with risk factors, aspirin therapy may increase risk of
hemorrhagic stroke.[1] Stroke accounts for nearly one third of all
cardiovascular events in women. Therefore, until we really have an evidence
base to suggest that aspirin lowers overall cardiovascular mortality, there
isn't a lot of enthusiasm to use it right now.
Dr Cauley: The Women's Health Initiative and the Heart and Estrogen
/Progesterone Replacement (HERS) studies both use conjugated equine estrogen (CEE)
and medroxyprogesterone acetate (MPA). What about some of the other forms of
HRT? My understanding is that they're now being used more frequently.
Dr Mosca: The world literature still largely pertains to CEE and MPA. One
school of thought exists that different estrogens and progestins may exert
different cardiovascular effects. In particular, there is a lot of enthusiasm
in Europe over 17-beta estradiol. Last year, Hodis presented an estradiol
study showing a reduction in the progression of corroded intima-media
thickness.[2]
In terms of progestins, there is a lot of controversy surrounding whether
or not one progestin might be better than another. The literature is quite
mixed. The take-home message is that we don't know whether or not one form of
estrogen and/or progestin is better. It's worth pursuing these studies to see
if we can develop a database that qualifies for a larger-scale critical
outcome study.
Unfortunately, the long-term epidemiologic studies of secondary prevention
have been minimal. So, in this case, epidemiology data seem to be consistent
with the randomized trials. And there is no primary prevention outcome study
yet.
Depression and CVD.
Dr Nolan: I see a lot of symptomatic depression in postmenopausal women,
which is also a considerable risk factor.
Dr Mosca: Indeed. Although the Enhancing Recovery in Coronary Heart Disease
[ENRICHD] trial, the first major randomized, clinical trial to test treating
depression and low social support with psychosocial intervention, did not show
a reduction in cardiovascular endpoints, there was a statistical improvement
in depression and social isolation.[3] There is great opportunity
for us to make sure that women get appropriate treatment for these problems. I
suspect that depression, anxiety, and social isolation are major contributors
to CVD for women.
Dr Nolan: Our community is now really looking for depression and treating
it. Dr Johnson, you and I were talking about using selective serotonin
reuptake inhibitors (SSRIs) for chronic pelvic pain and premenstrual
dysmorphic disorder several years ago.
Dr Johnson: Yes, and at present, a lot more practitioners are now
prescribing SSRIs for these and other hormone-related psychological mood
disorders.
Dr Dore: Thanks to the higher level of social acceptance than these agents
once had, patients as well as clinicians are now more willing to discuss and
use these therapies.
Dr Nolan: Indeed, and over the years, I'm finding less and less reluctance.
Twenty years ago, a colleague of mine said CVD was related to chronic stress
causing damage to the arterial walls. I thought it was an interesting theory,
and I still think there may be an element in there somewhere.
Dr Mosca: Absolutely. The role of psychosocial markers in CVD is now very
prominent. A number of studies have examined, for example, type A personality
as a risk factor for cardiovascular events. Unfortunately, most of this
research has been conducted in men. Only in recent years have even the major
studies begun to collect information about how personality traits and
reactions to environmental stress affect heart health. We all suspect that
these factors are major contributors to CVD in women.
Another issue is the multiplicity of the family and societal roles women
now have. For many years, women have been the caretakers of their spouse and
children; now, they are often also in the "sandwich role" of
caregiver for a parent, while also working outside the home. How the
psychosocial environment contributes to CVD remains to be determined. Common
sense tells us that it doesn't bode well to have competing priorities. Because
of these responsibilities, many women tell us that they don't have time to
exercise or to go to cardiac rehabilitation, for example. They feel their need
to care for others is greater than that to care for themselves.
When the World Trade Center disaster occurred, we set up a heart attack
prevention center downtown. We did it because severe emotional stress leads to
a host of responses from the body: Viscosity is increased, fibrinogen levels
are increased, blood pressure is increased, and cholesterol is even increased
in some studies. All of these markers can acutely affect risk among
individuals who have unstable plaque.
Here is another example: There tends to be about an eight fold increase in
the risk of cardiovascular events in the 2 weeks following earthquakes and
other natural disasters. For about 6 months, cardiovascular mortality rates
remain doubled.
Hormone Alternatives.
Dr Dore: Nutraceuticals are very important to my patients. Have any trials
looked at soy or red clover?
Dr Mosca: The soy data have been mixed, also. Some surrogate endpoint
studies, such as those on endothelial function, have been positive.[4]
Others have been neutral.[5] Many women like the idea of using a
natural form of estrogen rather than a pharmaceutical compound. I have
concerns about that, because I don't know exactly what they're getting or what
it's doing to their blood vessels. There's evidence that soy, when it's
replacing animal protein, can lower cholesterol.[4] Again, whether
these intermediate endpoint data are going to translate into beneficial
clinical outcomes remains to be determined. It would be very nice if we could
see a well-conducted trial of soy in postmenopausal women. In terms of our
recommendations, it's premature right now to say that soy has a cardiovascular
benefit.
The Faculty Speaks: A Roundtable Discussion on Postmenopausal Health Care
Thomas E. Nolan, MD, MBA; Susan Johnson, MD; Robin Dore, MD; Jane Cauley,
DrPH; Lori Mosca, MD, MPH, PhD
Bone Degeneration
Awareness is the key to preventing osteoporosis. Here, Robin Dore, MD,
author of "Osteoporosis: Focusing on Fracture and Key Risk Factors,"
discusses this disease with the other faculty
members, offering critical information on monitoring, prevention, and
treatment.
Dr Dore: My primary goal in speaking to patients and physicians is to
increase their awareness of osteoporosis prior to the fracture so we can
prevent those fractures from occurring. Luckily, we have many therapies for
preventing and/or treating these patients, including alendronate, raloxifene,
risedronate, and calcitonin. Estrogens no longer have the indication for the
treatment of osteoporosis as there has been no clinical evidence indicating
that estrogen reduces the risk of fractures. In the future, maybe next year,
we will have therapies that are anabolic and actually increase bone formation.
So what we can do today is identify the patients who are at risk, start them
on therapy, and have them be ambassadors of bone health.
We need to constantly emphasize to adolescents the importance of building
peak bone density -- through calcium intake, weightbearing exercise, and not
smoking.
Screening.
Dr Cauley: The National Osteoporosis Society guidelines do not recommend
follow-up bone mineral density (BMD) measurement once a woman is on therapy.
Why wouldn't you need them?
Dr Dore: The National Osteoporosis Foundation guidelines on vertebral
fractures assume that the patient has osteoporosis. But a malignancy [eg,
multiple myeloma, primary malignancies of bone] can cause those vertebral
fractures as well. A patient could have a trauma as a child and not remember
having a vertebral fracture.
One of my patients had a vertebral compression fracture at T10. She was on
long-term thyroid replacement, had never taken estrogen, was at risk of
falling, and had a positive family history of osteoporosis. If I had treated
her, she would have taken therapy needlessly, because she had a normal BMD. As
it turns out, she had fallen down a flight of stairs when she was 12 -- that
was when the fracture occurred.
Dr Cauley: Bone mineral density is a good screening tool. It identifies
people at risk for fracture. But it's not perfect. A patient with a BMD higher
than -2.5 SD can still have a hip fracture.
Dr Dore: Indeed, the problem with the World Health Organization criteria
for osteoporosis is that age and prior fracture are not taken into account. I
put age first, fracture history second, and then T-score. So, if a person has
a T-score of -1.8 but has a hip fracture, I would certainly treat her. I
advise that these guidelines not be used too strictly.
Unfortunately, I've had many patients referred to me for treatment of
osteoporosis because they've had a fracture, and it turns out to be a
malignancy. I always evaluate a patient for malignancy if she's had a fracture
and the assumption has been that it's osteoporotic.
Dr Johnson: Do we know what the prevalence is of morphometric fractures in
women based on their BMD? For example, in women with BMD above -2.5 SD and who
are 70 years old?
Dr Dore: In 80-year-old patients, the prevalence is 44%.[6] With
regard to BMD measurements, a great disparity exists among different machine
manufacturers. Therefore, I always recommend using the same machine every time
if possible. If that's not possible, the next best thing is to use a machine
from the same manufacturer.
Troubleshooting.
Dr Nolan: What about hyperthyroidism and overuse of thyroid replacements?
Dr Dore: Three abstracts at the World Congress of Osteoporosis last year
looked at these data. They showed that between 4 and 9 years of having a
normal ultrasensitive thyroid-stimulating hormone level, some bone loss
occurred. Other recent data showed that one of the greatest risks for hip
fractures was longterm thyroid replacement.[7] Thus, it appears
that the endogenous thyroid hormone acts differently than taking either
exogenous thyroid or exogenous corticosteroids in this regard.
The issue of frozen bone also came up, surrounding the fact that there was
increased
tetracycline labeling along the surface of the bone in patients who were
taking both alendronate and estrogen. This was not the case in patients taking
residronate and estrogen. Also, alendronate has a half-life of about 10 years
in bone and residronate is about 400 days.
The Faculty Speaks: A Roundtable Discussion on Postmenopausal Health Care
Thomas E. Nolan, MD, MBA; Susan Johnson, MD; Robin Dore, MD; Jane Cauley,
DrPH; Lori Mosca, MD, MPH, PhD
Safety of Postmenopausal Interventions
Any discussion of postmenopausal health interventions warrants the
inclusion of their respective safety issues. In this section, the participants
focus on the use of SERMs and estrogen replacement in a variety of health
scenarios.
Dr Cauley: Between 2005 and 2007, we can expect a large amount of data to
surface regarding breast cancer prevention, SERMs, and HRT. We will then have
a lot more information on the safety of these interventions.
Dr Dore: If a woman does have some vaginal dryness and is on raloxifene,
there are studies demonstrating that topical estrogen use is safe. Do you
agree?
Dr Johnson: That's an important point. I believe that vaginal estrogen can
be used in those women reasonably safely, now that we have available two forms
of delivery systems that don't lead to systemic absorption and therefore don't
lead to receptor competition. The estradiol ring (Estring) and
estradiol vagina tablet (Vagifem) are not absorbed systemically and can
be used safely in almost any patient if vaginal atrophy symptoms are present.
Dr Cauley: I just want to emphasize that raloxifene is not approved by the
US Food and Drug Administration for breast cancer prevention. The data from
the Multiple Outcomes of Raloxifene (MORE) study is, however, very exciting;
it didn't demonstrate simulation of the endometrium with raloxifene. Other
trials showed an increased risk of endometrial cancer with tamoxifen use,
particularly in patients older than 50. Therefore, in women with a uterus,
raloxifene has potential as an attractive therapy. Of course, we need more
data before we can recommend it for breast cancer.
Dr Dore: Raloxifene is not recommended to control vasomotor symptoms, such
as hot flashes, associated with menopause. However, it is a good option for
asymptomatic women who want to remain on preventive therapy.
If a 70-year-old patient is using HRT and her BMD is high, would you
recommend that she stop estrogen at that point because she's at increased risk
for breast cancer?
Dr Cauley: The American College of Obstetrics and Gynecology recently
recommended that after 5 years of HRT use, it is important to weigh the
benefits and risks of HRT for an individual woman. Use of the Gail Model for
Estimating Breast Cancer Risk can be helpful (Table). The major benefits of
HRT are symptom relief and prevention of bone loss; and risks include possibly
breast cancer, especially after long-term use and deep vein thrombosis. In
other words, I think she needs to reconsider why she is taking the HRT.

Table 1. (click
to enlarge) The Gail Model
Dr Johnson: For what reason is this 70-year-old patient continuing HRT? She
clearly doesn't need it for bones. Perhaps it will help with Alzheimer's
disease. She needs to understand that she is at somewhat increased risk for
breast cancer, merely by being on the hormones for 20 years, let alone her
high BMD. We have to be careful about how we explain those risks and benefits
to patients.
As I understand it, the Alzheimer's-estrogen connection began about 10
years ago with a few very small open trials. Patients with Alzheimer's disease
were given estrogen in an uncontrolled fashion and, after a few weeks, were
observed by clinicians to be thinking better. Later, epidemiologic studies
showed that women who had taken estrogen seemed to have about a 50% lower rate
of developing Alzheimer's disease. Mechanistic studies in the lab showed that
estrogen stimulated dendrite development. In theory, these factors explained
why estrogen might help to prevent Alzheimer's disease in women. Two years
ago, however, the first large, 1-year, randomized trial of estrogen vs placebo
in a group of women with early Alzheimer's disease was conducted and showed no
difference between the two groups. This study raised caution about the extent
to which we should believe those epidemiologic studies; after all, they have
the same kinds of biases with this disease as they do with others.
Dr Nolan: I've begun to focus more on study design and process, and when
higher cortical function is observed, to examine the tests that are used and
their reproducibility.
Dr Dore: Raloxifene is also being studied in the prevention and progression
of Alzheimer's disease. One group looked at five different tests of cognitive
functioning and showed that, using two of them, a significant improvement was
seen.
The Faculty Speaks: A Roundtable Discussion on Postmenopausal Health Care
Thomas E. Nolan, MD, MBA; Susan Johnson, MD; Robin Dore, MD; Jane Cauley,
DrPH; Lori Mosca, MD, MPH, PhD
Conclusion
Dr Nolan: I'd like to extend my thanks to everyone for joining me today and
sharing your thoughts about postmenopausal health. This lively conversation is
sure to have value to our readers in their pursuit of optimal patient care.
The Faculty Speaks: A Roundtable Discussion on Postmenopausal Health Care
Thomas E. Nolan, MD, MBA; Susan Johnson, MD; Robin Dore, MD; Jane Cauley,
DrPH; Lori Mosca, MD, MPH, PhD
References
- Sanmuganathan PS, Ghahramani P, Jackson RP, et al. Aspirin for primary
prevention of coronary heart disease: safety and absolute benefit related
to coronary risk derived from meta-analysis of randomised trials. Heart.
2001;85(3):265-271.
- Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of
atherosclerosis: a randomized, double blind, placebo-controlled trial. Ann
Intern Med. 2001;135(11):939-953.
- Berkman LF, Jaffe AS, for the ENRICHD Investigators. The effects of
treating depression and low social support on clinical events after a
myocardial infarction. Presented at the AHA 74th Annual Scientific
Sessions. Anaheim, Ca: 2001.
- Yildirir A, Tokgozoglu SL, Odunco T, et al. Soy protein diet improves
endothelial function and lipid parameters. Clin Cardiol. 2001;24(11):
711-716.
- Mosca L, Collins R, Herrington DM, et al. Hormone replacement therapy
and cardiovascular disease: a statement for healthcare professionals from
the American Heart Association. Circulation 2001;104(4):499-503.
- McClung MR, Geusens P, Miller PD, et al. Effects of risedronate on the
risk of hip fracture in elderly women. N Engl J Med. 2001; 344(5):333-340.
- National Osteoporosis Foundation. Physician's Guide to Prevention and
Treatment of Osteoporosis. 2000.
Faculty and Disclosures
Authors
Jane A. Cauley, Dr.P.H.
Associate Professor of Epidemiology, Graduate School of Public
Health, University of Pittsburgh.
Disclosure: Research Support: Eli Lilly and Company; Pfizer Inc;
Merck and Co, Inc.
Consultant: Eli Lilly and Company.
Speakers' Bureau: Eli Lilly and Company; Procter and Gamble
Pharmaceutical.
|
Robin K. Dore, MD
Clinical Professor of Medicine, University of California at
Los Angeles.
Disclosure: Research Support: Aventis; Eli Lilly and Company;
Merck and Co, Inc.
Consultant: Aventis; Eli Lilly and Company; Merck and Co, Inc.
Speakers' Bureau: Aventis; Eli Lilly and Company; Merck and Co,
Inc.
|
Susan Johnson, MD
Associate Professor of Medicine, University of Iowa College of
Medicine, Iowa City.
Disclosure: Research Support: Wyeth-Ayerst Pharmaceuticals.
Speakers' Bureau: Wyeth-Ayerst Pharmaceuticals; Eli Lilly and
Company; Pfizer Inc.
Consultant: Eli Lilly and Company.
|
Lori Mosca, MD, MPH, PhD
Associate Professor of Medicine, Division of Cardiology and
Division of Preventive Medicine and Nutrition, Columbia
University College of Physicians and Surgeons, New York, NY.
Disclosure: Consultant/Speakers' Bureau and/or Advisory Board:
Abott Laboratories; Bayer Corporation; Cholestech Corporation;
Kos Pharmaceuticals; Eli Lilly and Company; Merck and Co, Inc;
Novartis Pharmaceuticals Corporation; Organon; Pharmacia and
Upjohn; Reliant; Wyeth-Ayerst Pharmaceuticals; Pfizer Inc;
Sankyo.
Research Support: Organon; Pharmacia and Upjohn; Reliant;
Wyeth-Ayerst Pharmaceuticals; Pfizer Inc; Sankyo; Vasocor.
|
Thomas E. Nolan, MD, MBA
Professor of Obstetrics and Gynecology; Head, Section of
General Obstetrics and Gynecology, Louisiana State University
School of Medicine, New Orleans.
Disclosure: Speakers' Bureau: Wyeth-Ayerst Pharmaceuticals; Eli
Lilly and Company.
|
|






