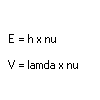The basics of formation of lines in a stellar spectrum.
Here I will discuss the formation of absorption or emission lines in the spectrum astronomers take from stars or, of course, our sun. It's basic for advanced undergraduates or starting graduates in astrophysics.

Light
We are all familiar with the sight of a rainbow. Beautiful colors from dark violet to bloody red. But what does it mean? We all know it takes sunlight and rain (or something more idyllic like a waterfall) to get a rainbow. Let's start with the colours first.
We normally interpret sunlight as being "white". However, anyone who ever made a colour-drawing knows that combining different colours gives more colours in return. (well, everybody who went to school knows that..) The white light of the sun really consists of a combination of a countless amount of colours. Leading a ray of sunlight (what a ray is, is as yet not clear) through a prism breaks the white light into it's different colours. The raindrops do the same. The rays of different colours change direction upon entering the raindrop, again upon hitting the "backside" of the drop and a last time when exiting the drop again. And the way in which the rays change direction depends on the colour.
So what is colour exactly?
Colours
Colours are in fact only interpretations of our brains. (But then again what isn't?) The true physics behind colours is in the properties of the rays themselves. There are two ways we can look at lightrays. Either as waves like the waves you see when you throw a stone in water, or as particles like little marbles, which are called "photons", (At first they were referred to as "quanta" by Max Planck and later Albert Einstein in the beginning of the twentieth century.) but then the word "rays" is maybe not appropiate, since we don't call electrons going through a wire a ray but a current, so maybe we should call lightrays lightcurrents when looking at them as a rain of photons.
Anyway, looking at them as waves it is most easy to understand the phenomenon of colours.
Anyone familiar with running waves (maybe from school) knows that there's something called "frequency". That has nothing to do with the velocity of the waves in a rigorous sense, but only with the amount of wave-tops that pass in a certain time-interval or fit in a certain space. (So, we can either squeeze the tops towards each other, while keeping the velocity of the moving wave the same, or keep the distances between two tops the same and make the wave move faster.)

See the picture above? One "wavelength" is the length after "one top up" and "one bottom down" denoted by the lambda. (In fact, in the picture it's denoted as the distance between to "tops", but check that this is the same distance as my definition.) So here 6 (cm). The shorter the wavelength, the more wavelengths fit in a certain time-interval or a certain space. So the shorter the wavelenth, the bigger the frequency. Now, violet light has a very large frequency, so, a small wavelength and red has a longer wavelenght. That explains the notion of colour. It is just another word for frequency. From large to small frequency: Purple (violet), blue, green, yellow, orange, red. JUST REMEMBER THAT FREQUENCY AND WAVELENGTH ARE INVERSELY PROPORTIONAL. (So increase one and the other decreases.)
In words of the particles, photons, it is somewhat different. Here we say that particles with high energies are on the blue side of the colours, while particles with low energies are more red. So, the energy of a light-particle is proportional to the frequency and inversely proportional to the wavelength.
Or In formulae:
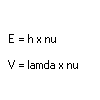
Which say that the energy of one of the photons is a constant, h called the constant of Planck, times the frequency of the particle. (which is strange, but which is explained here: )
The physics of the small.
The next says that the wavelength and frequency of a wave are connected by the speed the wave is moving. The velocity of the wave is the wavelength times the frequency.
The Processes behind Light
Now that we know that a certain colour comes with a certain wavelength which is associated with a certain energy, we can start to think about the processes which are responsible for the formation of light. A lot of info can be found on this page.
Some quantum-physics behind the formation of a photon.
After heaving read the above and knowing that electrons can jump between different energy-levels in an atom, we can explain the formation of a certain line in a spectrum. We simply need an electron in a high level, falling down to a lower level and releasing the energy in the from of a photon. This photon thus carries the exact amount of energy which corresponds with the difference in energy of the electron in the corresponding orbits.
A simple experiment can be done with a candle and some normal kitchen salt. Just light the candle and drop some of the salt in the flame. Did you see the difference in colour? What happens is that certain electrons in certain orbits of certain atoms in the salt-crystals are pushed in a higher orbit when particles in the flame collide with them. Those particles in the flame, obviously, have a pretty high kinetic energy. The electrons then fall back and release the difference in energy in the form of a photon with a very specific frequency, (The particles in the flame have only enough energy to push the electrons in the salt to ONE other energy-level. That's why we see only ONE frequency.) so we see one colour. This would give a sharp line in a spectrum taken from the candle. This is a so called emission line.(In fact it's a transition in sodium atoms. Normal salt consists of a crystal-structure of Sodium-Chloride. The salt first evaporates and the transition takes place with the sodium atoms. The same colour can be seen in Sodium Highway lights.)
However, the opposite is also possible. Imagine a candle emitting light of all different wavelengths. Now imagine we put a cloud of Sodium atoms between the candle and our eye. What happens?
We know that the photons with the right frequency have a change (quantummechanically) of pushing an electron in an orbit of a sodium atom in a higher orbit. This electron will eventually fall back and remit a photon. So netto, nothing really changes. (Assuming there is only one way back for the electron. In more complex situations an electron pushed up, might fall back via many steps releasing many photons with different energies. The total energy of all those photons must equal the energy of the incoming photon. This is a simple consequence of the law of conservation of energy. However, he who has read the page on atom physics thoroughly might say that the energy-levels in the atom are not infiniteally sharp, but are spread due to Heisenberg's uncertainty principle. Therefore there is a possibility that the total energy is not the same. The question whether or not this is true will be discussed thoroughly further on. Just think about it for a minute before continuing. What would be the consequense on a bigger scale, if the energy would not be conserved?)
However, you must realize that the photon which is re-emitted can be re-emitted in every direction! So, the amount of photons which go in the direction of the observer at that specific wavelength is smaller than the same amount at different wavelengths where no absorption takes place. We see a darker line in our spectrum! This is a so called absorption line.
Here are some examples of spectra with emission lines and/or absorption lines. Observe that they are not infiniteally narrow!(Of course this is also because there are no human devices which can measure with infinite precision. And this is not directly a consequense of quantum-mechanics, but more of a limitation to our craftmanship!)

The lines hydrogen produce. What is the side of highest energy? What corresponds with the biggest distance between two orbits? Check the transitions page and look at the energy-term diagram. Try to find the some of the possible energy-transitions in this diagram.

Same for helium. It is an atom with two electrons. Therefore it is more complex and has more possible transitions.

Same for carbon. It has 12 electrons. Even more complex.

This picture shows the absorption lines in a spectrum of the sun. The first to have reported these lines was a scientist called Fraunhofer. That's why these absorprion lines are still known as "Fraunhofer Lines". The drawing above shows a graph of the Planck curve. It is a representation of the intensity of the continuum of the sun. It will be discussed below.
So, we now know that lines can be formed by transitions in atoms, when electrons jump between different energy levels. We also know that the oppisite is also possible; photons from a source are absorbed and re-emitted in different directions, so that the intensity of the source at that particular wavelength decreases. The first is called an emission line, the latter an absorption line, also known as Fraunhofer lines. (At least in a solar spectrum.)
But take a look at the last picture. There are an infinite amount of colours there. Do you really think I want to make you believe those lines are all the result of atomic transitions?
No, in case of the sun, it goes like this: there is a background continuum of light, upon which the different transitions, be it an absorption or emission line, are superposed. But what is a "continuum" and, if it doesn't consist of light from atomic transitions, how is it formed?
I will come to that now.
The continuum
There are many ways light can be formed, without the need of atiomic transitions between two energy levels in an atom. This automatically means that the energy of the photons involved in those processes is not sharply determined. Normally there is a maximum energy the photons can get in the processes, and there is a certain law by which the lower energies are divided. I will mention some of the processes involved in forming the continuum.
Bound-Free Processes
The simplest way of forming a continuum are the so called bound-free processes. This are processes where an electron is kicked out of an energy level with enough energy to leave the atom completely. This is called Ionisation. In a term scheme, it means that the electron goes from a bound level all the way up and beyound all the existing bound levels. All the energy it gets is therefore divided over de energy needed for the electron to be ionised and the extra kinetic energy the electron gets after being ionised. This extra energy only depends on the energy of the incoming photon, which kicked it out of it's bound level. (Until now, I assumed that only atoms can kick electrons out of orbits. However, it can also be done by other massive particles in "collisions".) Now, this means that any photon with an energy higher than the ionisation energy can be absorbed. This causes a large area of the spectrum to be darker.
The other way round, when an electron like that returns, it will release a photon with a total energy of the ionisation energy plus the extra kinetic energy it had. So a lot of those electrons will cause a wide variety of photons, thus forming a continuum.
Free-Free Processes
All the free electrons are moving about in a great soup of particles. All those particles interact with each other. Now, when the amount of particles is high enough and there is a continuous interaction, the velocities of all these particles will follow a so called Maxwell-distribution. (This will be explained in the atom-physics section.) Most particles caryy a charge (like the electron carries a negative charge, called the elemental charge) and charged particles tend to influence each other by means of their electric fields. (the simplest form being the Coulomb attraction or repulsion. Again the basics of electrostatics will be discussed in the topic of atom physics) These forces tend to accellerate the particles, making them change direction, or speed up. One of the consequences of the theory of electrodynamics (Maxwell Theory) is that accelerated charged particles will radiate. So, that's another form of continuum radiation. The range of energies are set by the time the interaction has to accellerate the particles, thus on the kinetic energy of both particles, the charge, the mass difference, etc. etc. The mean velocity of the particles is set by the Maxwell distribution mentioned above and thus is the kinetic energy. (Yes, it is the same Maxwell!) This process is called "Thermal Brehmstrahlung".
Since there are a lot of particles, it is possible that they will collide in a more direct sense. During this collision electrons might be released, and caught back again, causing the release of photons of different wavelengts. Electrons might move from one atom to another, causing a difference in energy. (Called charge transfer.)
Magnetic Influence
When moving charged particles encounter a magnetic field (Magnetic fields are somehow more unclear than the concept of electric fields. Some will be explained in the same section as the concepts of electrostatics. However, remember that any "field" mentioned is just a mathematical tool to make the effect of magnets or charges visible.) they will start to spiral around the magnetic field lines. The exact direction and frequency dependence will be mentioned in the atom physics section, but there are generally two cases.
Cyclotron Radiaton
Charged particles with speeds much lower than the speed of light encounter a magnetic field line and are forced to move in a spiral around that field line. They are thus accellerated (accelleration is also change of direction even without actually speeding up!) and will radiate photons. They will radiate photons with a frequency exactly the same as their "spiraling-frequency". Normally, this frequency is in the so called radio region, which means that it has frequencies similar to the frequencies of radio signals. (Tens of megahertz, with one hertz meaning one complete wavelength or period per second. See the definition of wavelength above.)
Synchrotron Radiation
This is the same proces, but now only with particles moving at relativistic speeds, meaning that they are moving at speeds coming close to the speed of light. (Maybe there will be a section on relativistics soon.) The effect is the same, only because of relativistic effects, the beam of photons will be much narrower and bundled in the direction of the movement of the particle. (Normally electrons are the only particles showing synchrotron radiation, since other particles are usually too heavy to accellerate towards relativistic speeds.)
Below is a schematic picture of what happens. The frequencies are of a high variety, from infrared to X-ray.

Scattering Processes
Another important issue is the scattering of photons on particles. Photons might go near a particle and be scattered. There are many ways of photon scattering. They might be frequency independent, like Thompson scattering, or frequency dependent, like Rayleigh scattering. The latter causes the sky to be blue. Photons from the sun are being scattered by bound electrons in molecules in the air. This is frequency dependent and photons with a higher frequency are more scattered than photons with a lower frequency. That's why the sun is more red coloured and the sky is blue. The first takes place in fog, for example. Photons from the sun are scattered by very losely bonded molecules in the water damp and only change direction, independent of their frequency. That's why fog is white.
Even though these are continuum processes, all the light we see comes from the sun. That's why it is important to realize that even in the light we could detect coming from fog, or the light which would be reflected by your nose for example, still contains all the absorption lines shown in the fraunhofer picture above!


Broadening
Aborption lines are not infiniteally sharp, as alreay mentioned, because of the Heisenberg Principle. This is called "Natural Line Broadening". However, there are more thing which cause so called "line-broadening". The influences of particles on each other, trough collisions and the rest, causes the broadening of lines. This is called "Thermal Broadening". Also the speed of a particle emmitting light causes a line to be broadened. This is so called "Doppler Broadening".
There is another interesting quantummechanical effect, mentioned in the atom physics section, called the "Stark-effect". This is the effect where an electric field causes certain energy levels to split in several energy levels, with an identical but very small split. So an energy level is suddenly divided in three or so very close energy levels, causing a broadening of a line profile. (Observe that I'm starting to be less and less elaborate about words like absorption line profile broadening. It's becoming line profile broadening, which will turn in line broadening into broadening. Please be sure you understand what you're reading!)
Another effect is the Zeeman effect, where due to magnetic fields a profile splits in two parts, each having a distinct "polarization", one parallel to the field direction and one anti-parallel. So, this is also broadening. One absorption line becomes two, but the total "surface" it covers will be the same, so both branches are smaller, the sum being the "surface" in absence of magnetic fields. Below is one of the earliest pictures of this effect. Observe that it is in fact not as simple as I seem to be telling you!

Doppler-shift
There is only one more thing you need to know of. Have you ever heard a car passing you by? Or maybe even an ambulance? Did you ever realize the strange thing that happens to the sound of it? When it is moving towards you the sounds seems to be of a higher tone than when it is moving away. This is the "Doppler-effect" for sound-waves.
The same goes for light rays. When the source is moving away from the observer, the color moves to the red part, while when the source is moving towards the observer, the color moves to the blue part.
Now, what would happen with an absorption line, being formed in the solar atmosphere, while the Sun rotates around his axis, just like Earth is? On the left part for example, the atmosphere is moving towards us, while on the right part the atmosphere is moving away. So, when we take a spectrum from the left side of the sun the line is moved towards the blue, while lines observed from the right side tend to be moved to the red. This is important if one want to identify the line, by it's wavelength, with a certain transition and thus with a certain element!
Again, by taking an integrated image from the complete visible solar disk cancels the effect, but reduces ones resolution. The best way to do it, seems to be to take a spectrum from the middle of the sun, since at that position the atmosphere has no velocitiy towards or away from the observer, only at a right ange in comparison with the viewing direction. But this doesn't influence the frequency. (Just as an ambulance moving without gaining distance with respect of you has a constant tone.) But this is of course only a very small region and you can not get a lot of light from a small region, unless you take a picture over a longer period of time, which blurs the image. Here is an example of the effect of the movement of a light source on an absorption line.

So, now we know that a continuum is being formed in the solar atmosphere by many processes. Absorption and maybe emission lines are formed by transitions of electrons between different energy levels and those lines are broadened and shifted by different effects. Good. But how can we describe the photons travelling through the atmosphere, and what do absorption lines look like in a higher resolution and what do we in fact measure from the Sun? What does it mean to be looking at the sun at different wavelenghts? From how deep do the photons in fact come, before hitting our instruments? All these kind of very tricky questions will be discussed from here. It will be much more mathematical and more elaborate. If you've had enough bail out, wimp! If you want to know...keep on going!
Describing Light Rays
Ok, take a very large number of photons, all on their way of moving through space. Let's call this a light-ray. Let's assume we have a detector with which we are going to detect the photons. Assume the detector has a surface of detection called dA. Assume our detector is usuable for a certain frequency-range between f + df. Assume we are going to measure a certain amount of time dt and assume that we can measure light filling a certain part of the sky dW.
Check the picture first.
![]()
First notice that the light ray is falling on our detector area dA under a certain angle theta with the normal n of the area.
The first notion you need to know is the notion of intensity.
It is the proportionality factor between the total amount of enery detected and the area, time, frequency band, and part of sky the detector can manage.
It is referred to as I and shows like this:
![]()
It has the dimension of Joules m-2 s-2 hz-1 sr-1. Here sr is a starradian, which is the amount of surface of the sky covered by the the light=ray falling on the detector. The total sky (so both hemispheres, north and south) have a starradian of 4 times pi. When looking at the sky we see only one hemisphere, thus we cover a starradian of 2 times pi. What the definition of it is, is shown in this picture.
![]()